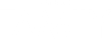90 70 90 Targets

Cervical cancer is a type of cancer that affects the cervix, which is the lower part of the uterus that connects to the vagina. This type of cancer is caused by the human papillomavirus (HPV) and can be prevented through early detection and vaccination. However, cervical cancer remains a significant health concern, with an estimated 600,000 new cases diagnosed worldwide each year. The general public needs to be aware of cervical cancer and its risk factors, symptoms, and prevention strategies being practiced in the world and in their own country. By increasing awareness, individuals can take steps to reduce their risk of developing cervical cancer and seek medical attention if they notice any symptoms.
Risk Factors
Certain factors increase a person's risk of developing cervical cancer. HPV is the most common risk factor for cervical cancer. HPV is a sexually transmitted infection that can cause changes in the cells of the cervix, leading to cancer. The risk of developing cervical cancer increases as women age. Most cases of cervical cancer occur in women over the age of 30. Women who smoke are more likely to develop cervical cancer than non-smokers. Women with an immune-compromised state, such as those with HIV or who have had an organ transplant, are at an increased risk of developing cervical cancer. Women with a family history of cervical cancer are at an increased risk of developing the disease.
Cervical cancer is highly preventable through early detection and vaccination. Eating a healthy diet and getting regular exercise can help boost your immune system and reduce your risk of developing cervical cancer. If you smoke, quitting can help reduce your risk of developing cervical cancer. Practicing safe sex can help reduce your risk of HPV infection, which can lead to cervical cancer.
WHO recommends an HPV DNA-based test as the preferred method, rather than a visual inspection with acetic acid (VIA) or cytology (commonly known as a 'Pap smear'), currently the most commonly used method globally to detect pre-cancer lesions. There are quite a few shreds of evidence and clinical trials are now available that confirm – “HPV-DNA testing detects high-risk strains of HPV which cause almost all cervical cancers”. Unlike tests that rely on visual inspection, HPV-DNA testing is an objective diagnostic, leaving no space for the interpretation of results. More access to commodities and self-sampling is another route to consider for better accessibility to the screening test even in remote places where healthcare specialists are not available.
In Viral Transport Medium the FDA/ WHO listed companies can keep the sample stable for several months.
WHO suggests that self-collected samples can be used when providing HPV DNA testing. On the basis of several countries’ experiences- women often feel more comfortable taking their own samples rather than visiting a service provider for having one for screening further. However, women need to receive appropriate support to feel confident in managing the process. Cervical cancer, although nearly 100% preventable, remains one of the most common cancers and causes of cancer-related deaths in women across the globe – especially in low-and middle-income countries.
The majority of cervical cancer cases are found in women who are unscreened or under-screened. There are many reasons women cannot or do not participate in a cervical cancer screening program –some of these barriers are abolished with the availability of a self-sampling system.
However, the crucial part of HPV (Human Papillomavirus) testing is the collection of a high-quality sample. This is typically done by taking a swab of cells from the cervix, which is then analyzed in a laboratory to determine the presence of HPV DNA. It is important to collect the sample properly and ensure that it contains enough cells for accurate testing, as a low-quality or insufficient sample can lead to false-negative results.
That is why it is strongly recommended to use a sampling device that is validated and enabled with a "control" mechanism that flags the integrity of the collected sample. Internal cellular control helps to avoid false negatives due to sample quantity and quality issues
ROCHE COBAS test had received the first approval for HPV Molecular testing from US FDA and similarly, Gardasil Vaccine from Merck & Co was the first brand to get approved. Both companies have very strong clinical trials to validate the accuracy and efficacy of their product.
Results Interpretation:
The results of an HPV test can be confusing, so it is important to understand what they mean. If the test is positive for HPV, it means that the patient has the virus. However, it does not necessarily mean that they have cervical cancer. Most HPV infections go away on their own within a few years and do not cause cancer. However, some HPV infections can persist and lead to the development of precancerous or cancerous cells.
If the test is negative for HPV, it means that the patient does not have the virus at the time of the test. However, it is important to remember that HPV can develop at any time, so regular screening is still recommended.
WHO Designs “With three key strategies and clear 2030 targets—an increase of HPV vaccination to 90%, twice-lifetime cervical screening to 70%, and treatment of pre-invasive lesions and invasive cancer to 90% (also known as the 90-70-90 targets)—this global call-to-action provides a roadmap to eliminate cervical cancer”.

-Roche Diagnostics
Cervical cancer is a preventable disease that affects millions of women worldwide. By increasing awareness of cervical cancer, its risk factors, symptoms, and prevention strategies, individuals can take steps to reduce their risk of developing the disease.
WE CAN END CERVICAL CANCER. Get Informed. Get Screened. Get Vaccinated.